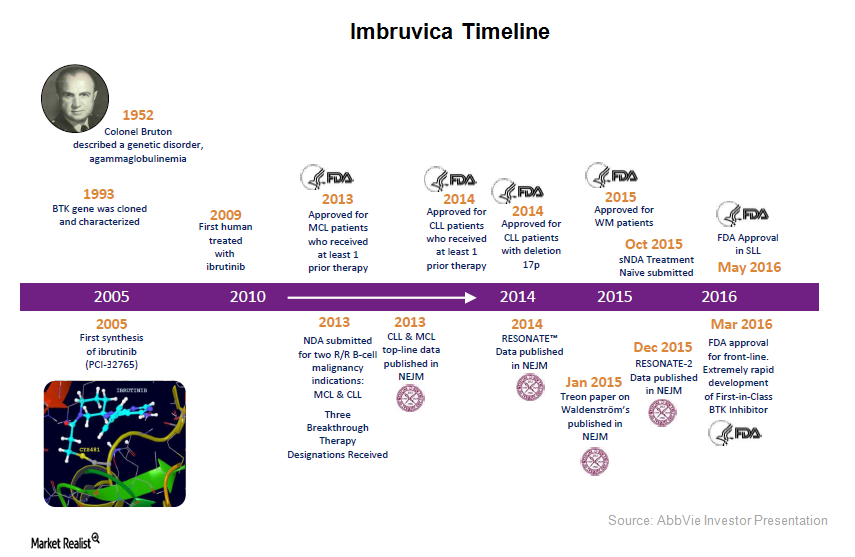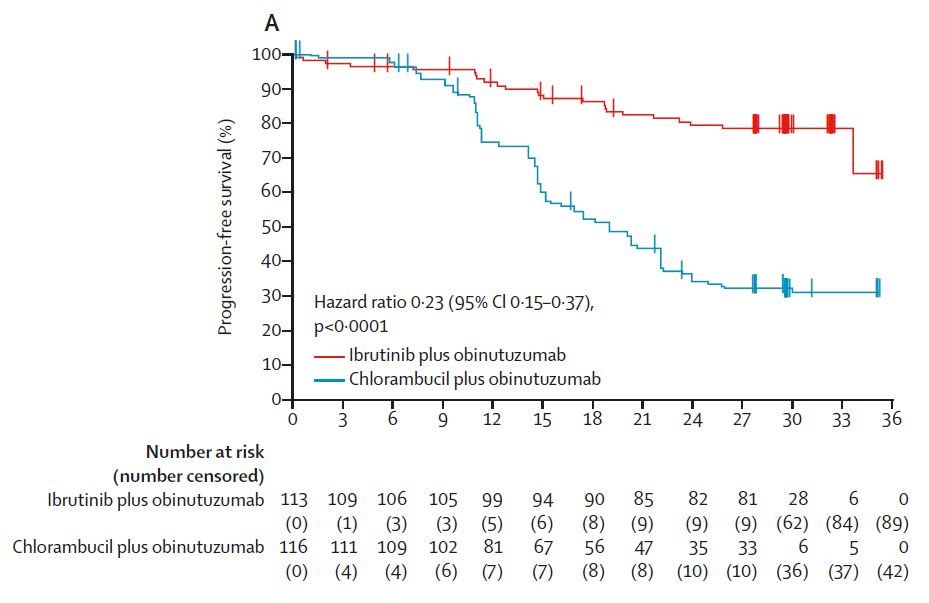
The Lancet Oncology on Twitter: "Presenting now at #ASH18: Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in firstline treatment of chronic lymphocytic #leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial ...

These highlights do not include all the information needed to use IMBRUVICA safely and effectively. See full prescribing information for IMBRUVICA. IMBRUVICA ® (ibrutinib) capsules, for oral use IMBRUVICA ® (ibrutinib) tablets,

These highlights do not include all the information needed to use IMBRUVICA safely and effectively. See full prescribing information for IMBRUVICA. IMBRUVICA ® (ibrutinib) capsules, for oral use IMBRUVICA ® (ibrutinib) tablets,

Supplemental Materials for Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study - The Lancet Oncology

Cheadaigh FDA na SA Johnson & Johnson / Imbruvica (Ibrutini) le haghaidh 11 thásc agus 6 le haghaidh cóireála leoicéime! - Nuacht tionscail - Nuacht - Hefei Home Sunshine Pharmaceutical Technology Co., Ltd

BeiGene nabs landmark FDA nod for Brukinsa, kicking off challenge against blockbuster Imbruvica | FiercePharma

Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): a phase 3, multicentre, open-label, randomised trial - The Lancet Haematology

Heavy water labeling in CLL patients before ibrutinib therapy: workflow... | Download Scientific Diagram

Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial - The Lancet Oncology