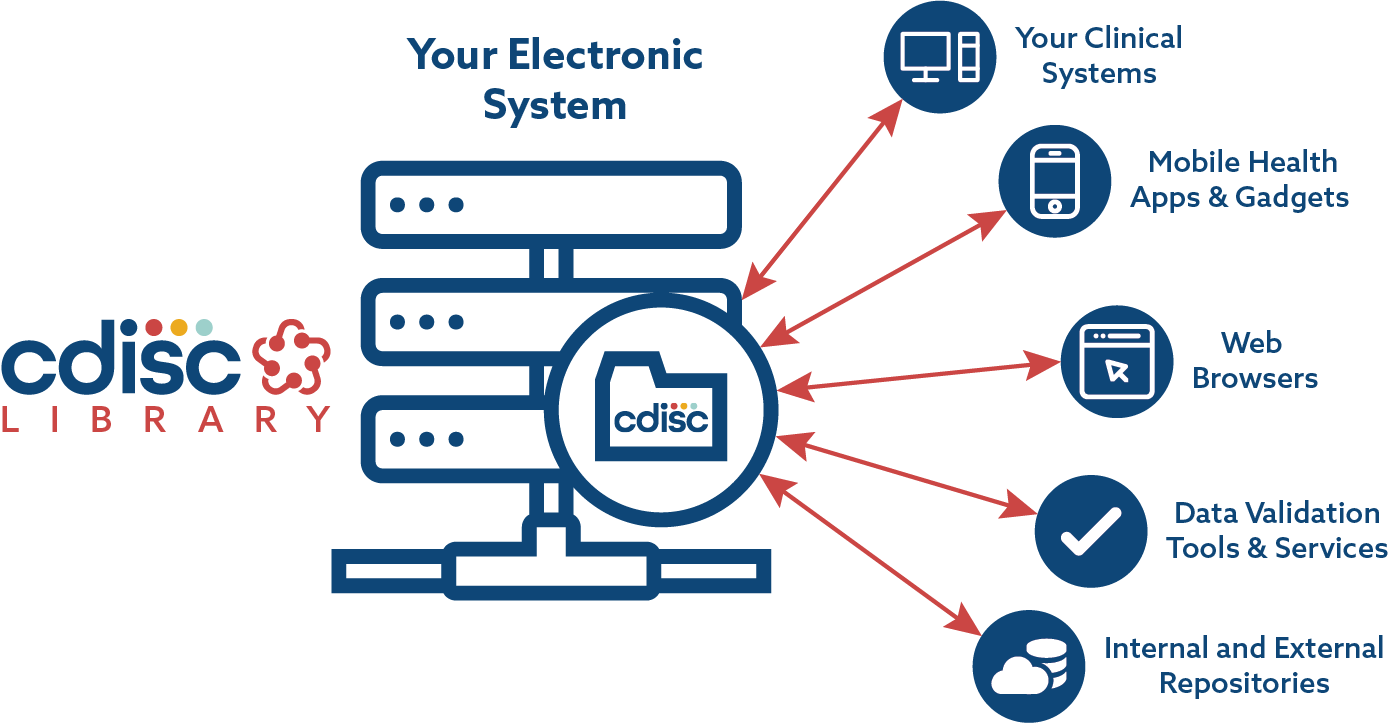
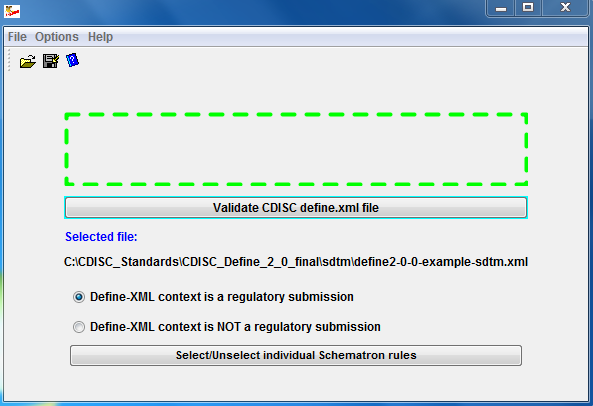
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/5-Figure4-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar

Sanofi-Aventis Experience Submitting SDTM & Janus Compliant Datasets* SDTM Validation Tools - Needs and Requirements - PDF Free Download
![Validation of ADaM datasets based on CDISC ADaMIG 1 Validation of ADaM datasets based on CDISC ADaMIG - [PDF Document] Validation of ADaM datasets based on CDISC ADaMIG 1 Validation of ADaM datasets based on CDISC ADaMIG - [PDF Document]](https://demo.documents.pub/img/378x509/reader022/reader/2020060905/5e8d8f8d792cc060124738d2/r-1.jpg)
Validation of ADaM datasets based on CDISC ADaMIG 1 Validation of ADaM datasets based on CDISC ADaMIG - [PDF Document]

A standard-driven approach for electronic submission to pharmaceutical regulatory authorities - ScienceDirect

A standard-driven approach for electronic submission to pharmaceutical regulatory authorities - ScienceDirect
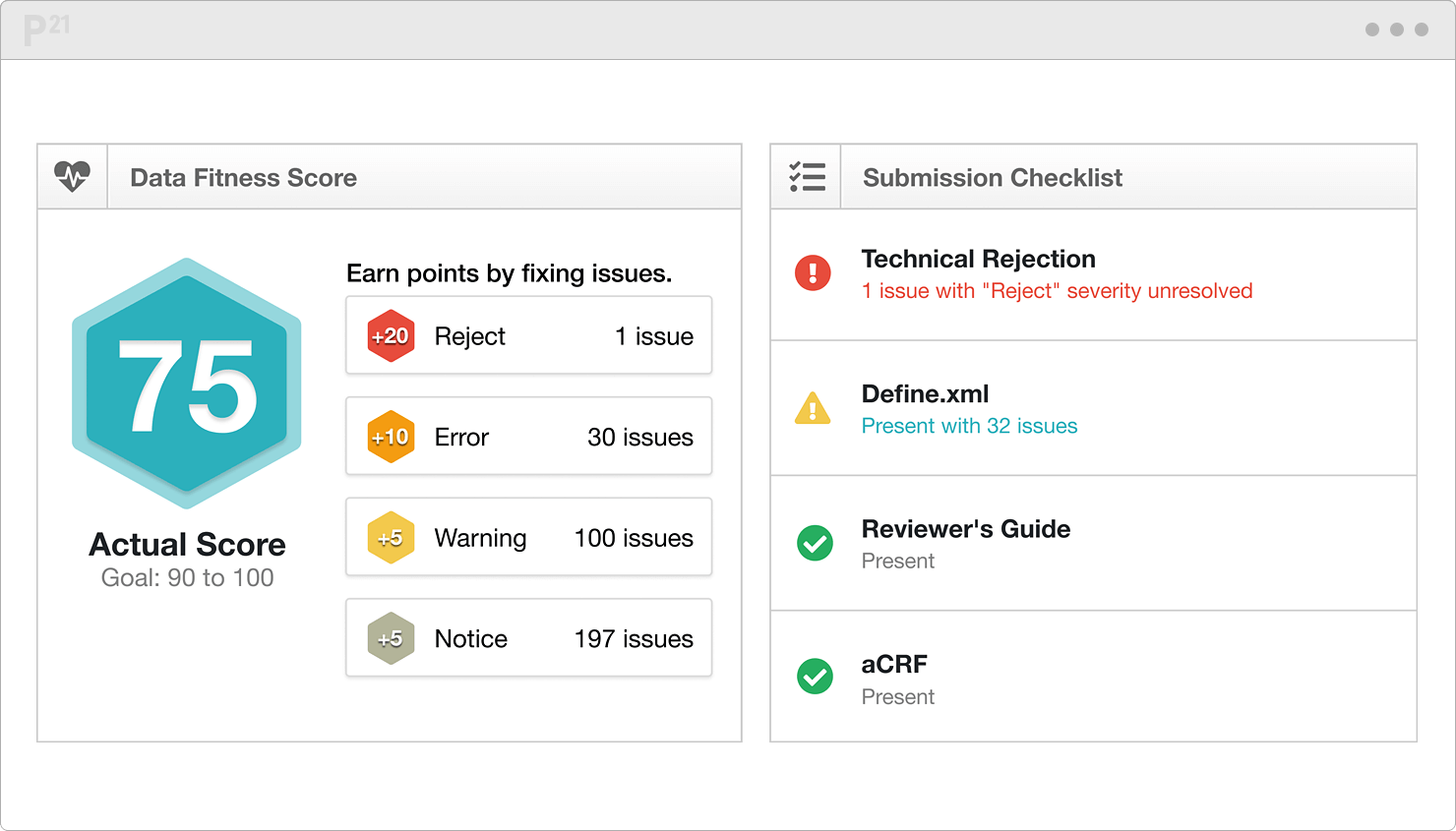
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/4-Figure3-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar
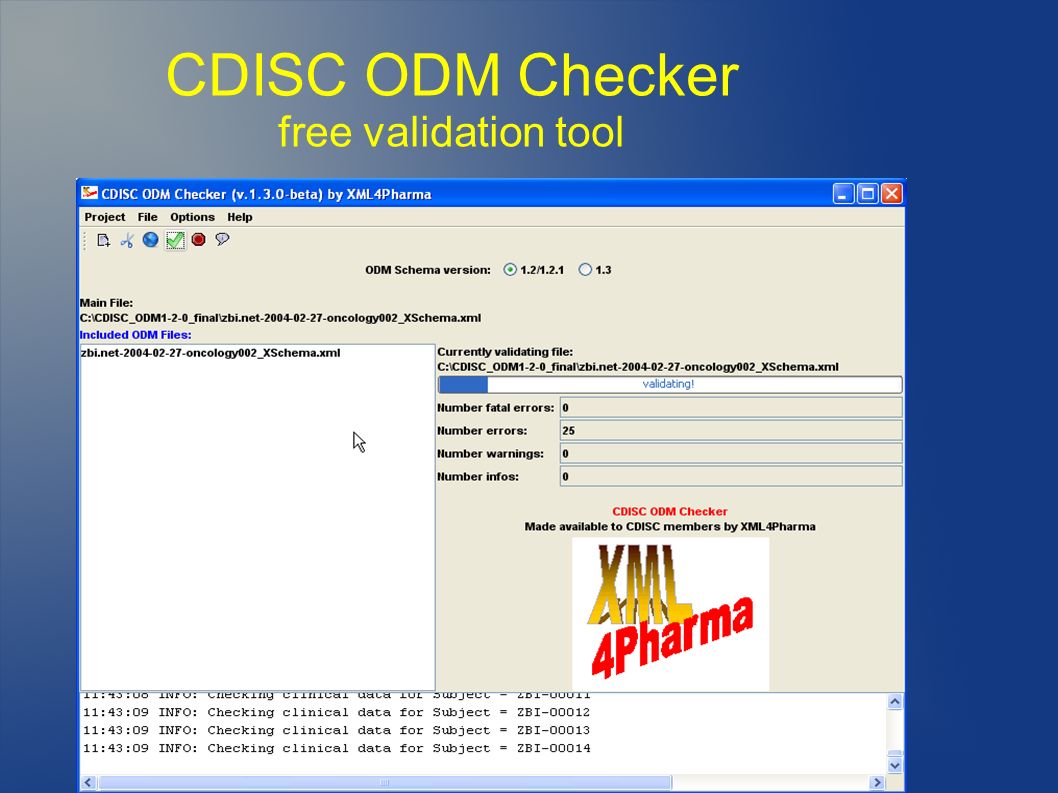
PLOS ONE: ODM Data Analysis—A tool for the automatic validation, monitoring and generation of generic descriptive statistics of patient data
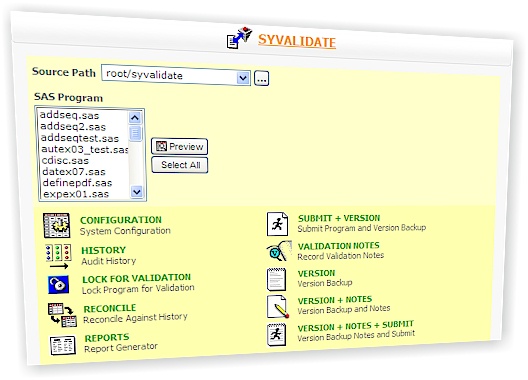
Sy/Validate is used for SAS Program Validation, SAS Program Validate, SAS Program Audit Trail, SAS Program Versioning, SAS Program Version Control, SAS Program Change Control, SAS Program Verification, SAS Program CFR Part
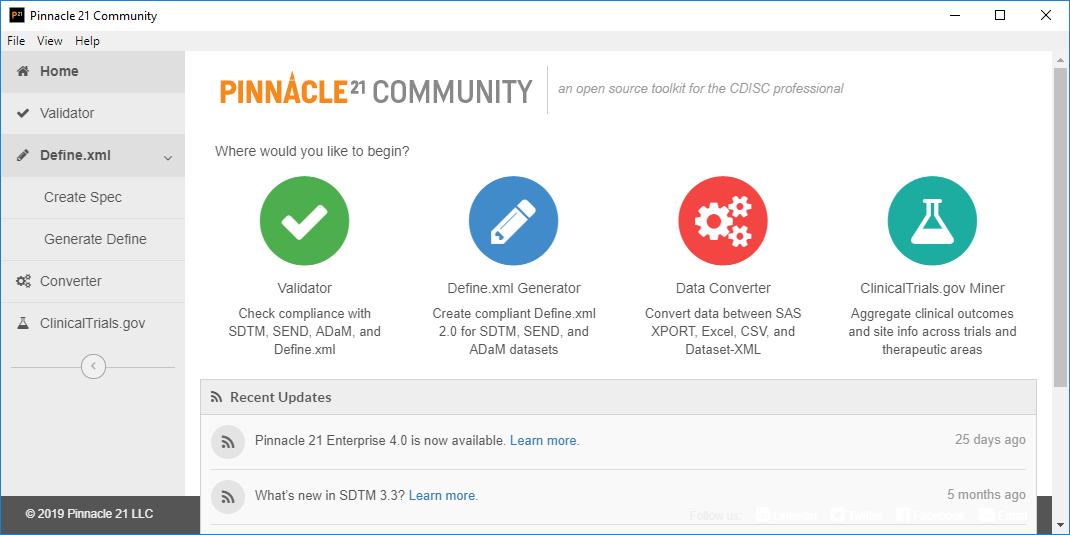
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/3-Figure2-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar