![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/5-Figure4-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar

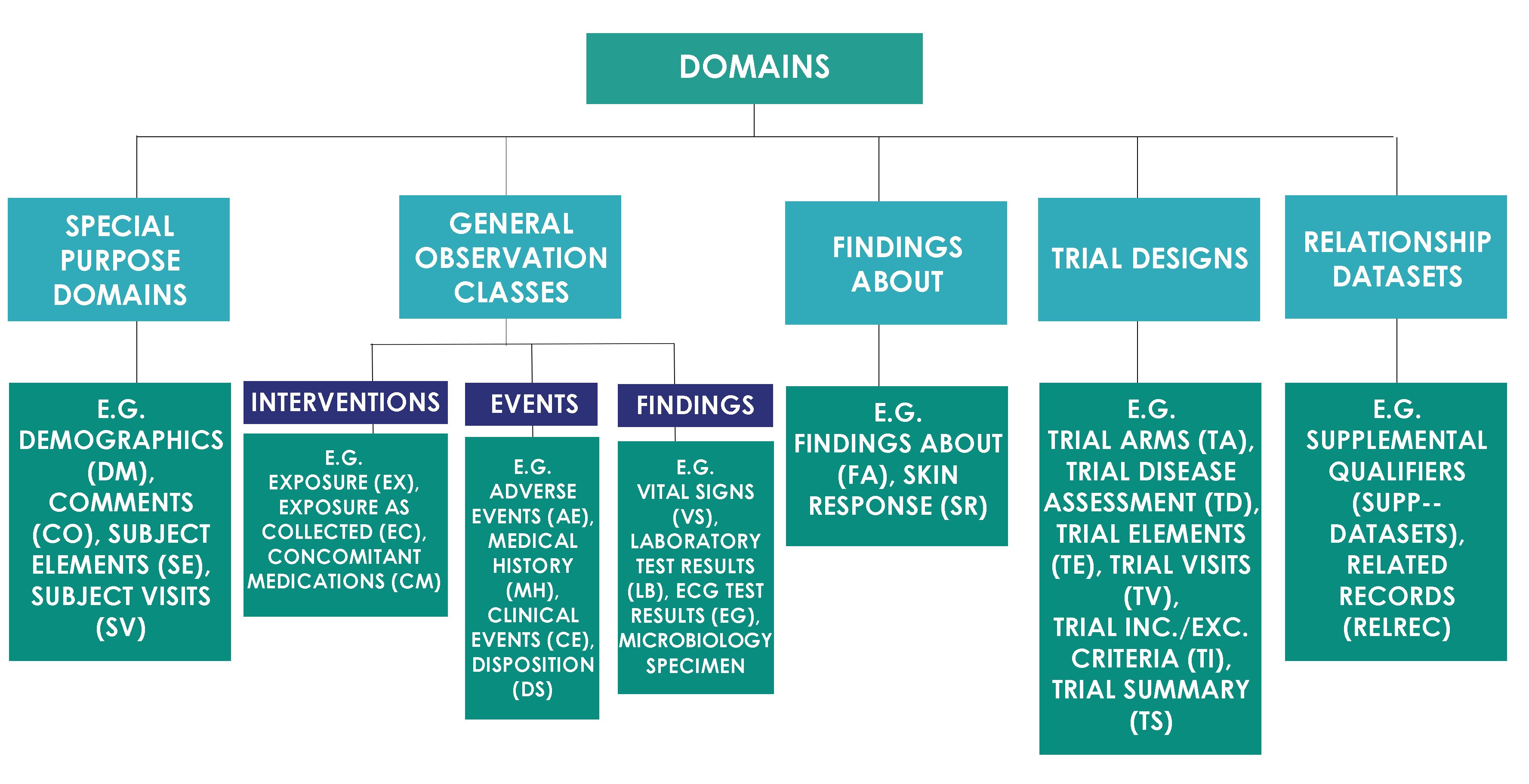
A standard-driven approach for electronic submission to pharmaceutical regulatory authorities - ScienceDirect
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/3-Figure2-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar

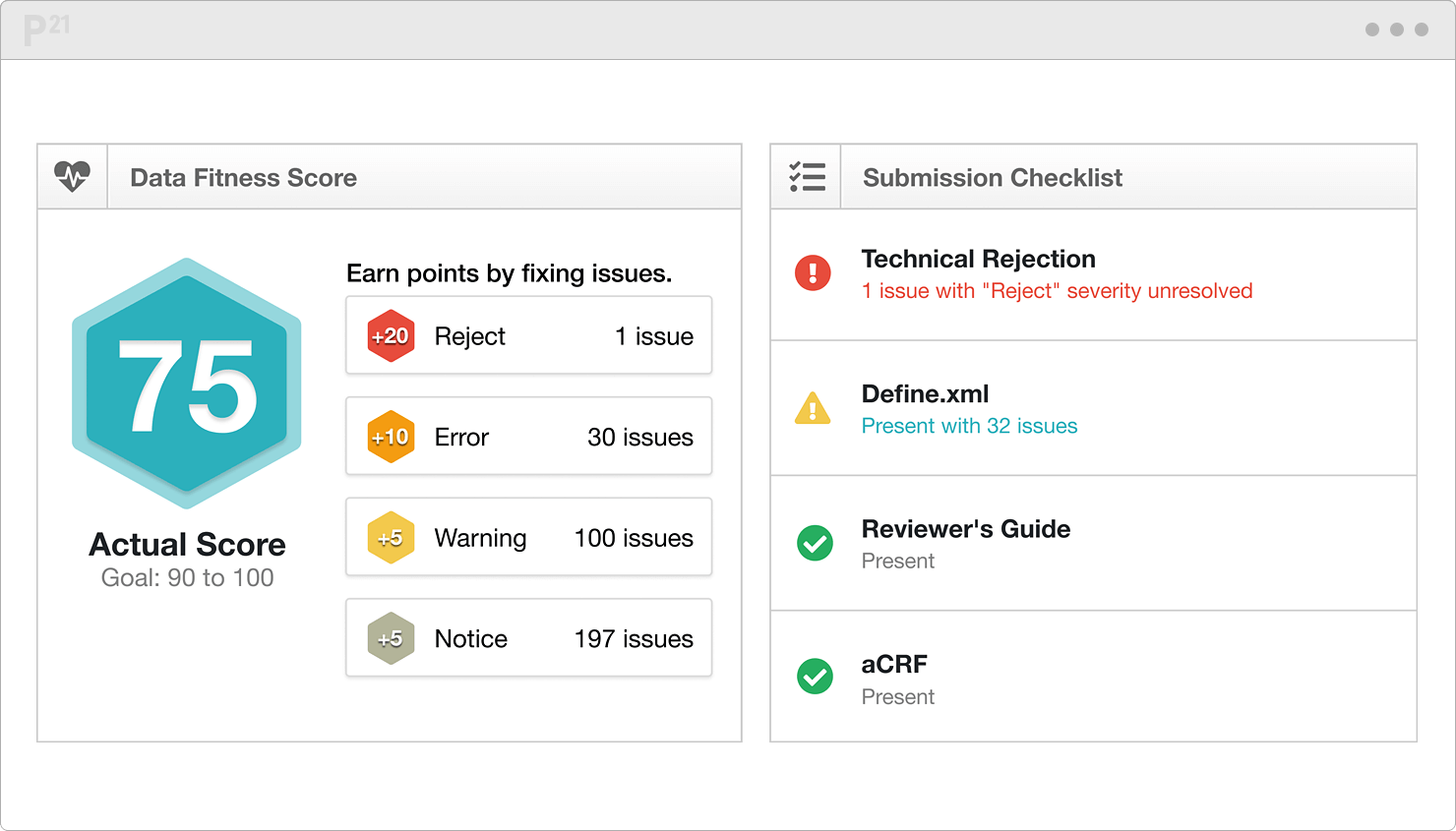
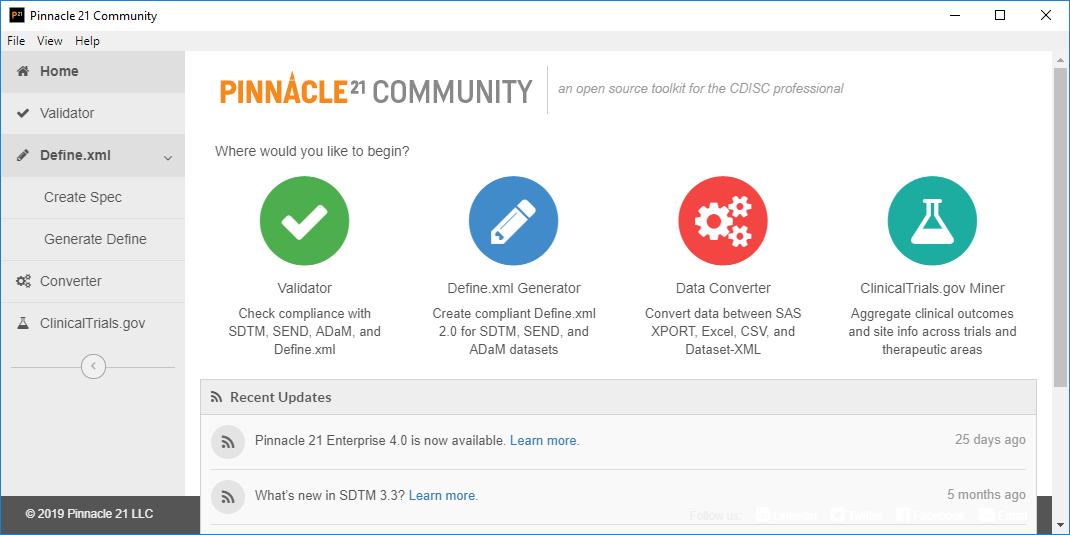
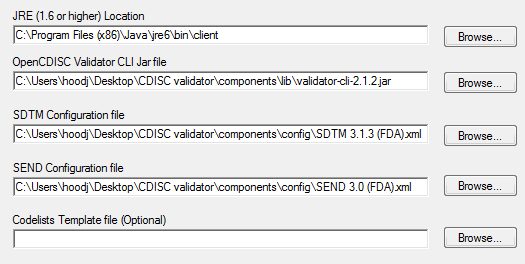
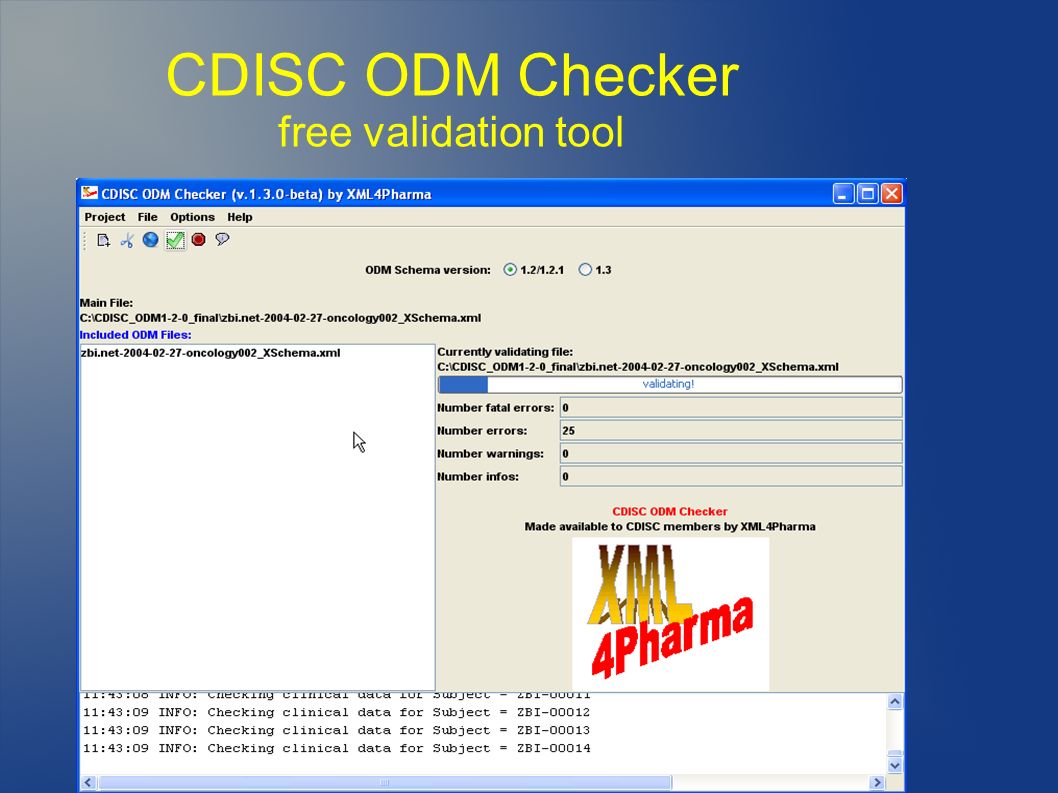
Sanofi-Aventis Experience Submitting SDTM & Janus Compliant Datasets* SDTM Validation Tools - Needs and Requirements - PDF Free Download
PLOS ONE: ODM Data Analysis—A tool for the automatic validation, monitoring and generation of generic descriptive statistics of patient data
![Validation of ADaM datasets based on CDISC ADaMIG 1 Validation of ADaM datasets based on CDISC ADaMIG - [PDF Document] Validation of ADaM datasets based on CDISC ADaMIG 1 Validation of ADaM datasets based on CDISC ADaMIG - [PDF Document]](https://demo.documents.pub/img/378x509/reader022/reader/2020060905/5e8d8f8d792cc060124738d2/r-1.jpg)